Drugs@FDA Search Simulator
Try this interactive tool to practice searching the FDA's Drugs@FDA database. Enter a drug name or active ingredient to see how the search results would appear and understand the differences between search methods.
Want to find out when a drug was approved by the FDA, who made it, or what the official prescribing information says? You don’t need to call the agency or dig through old PDFs. The Drugs@FDA database gives you direct access to every approved drug’s regulatory history - for free, and without signing up. It’s the most reliable source for official FDA drug records, updated daily since 1939. Whether you’re a pharmacist, a patient, a researcher, or just someone trying to understand your prescription, knowing how to use this tool saves time and cuts through misinformation.
What Is Drugs@FDA and Why Does It Matter?
Drugs@FDA is the U.S. Food and Drug Administration’s official public database for approved human drugs. It includes records for more than 20,000 drug products - from common antibiotics to complex biologics. Unlike websites that summarize drug info, Drugs@FDA shows you the original documents the FDA used to approve each drug: the full prescribing label, review reports from FDA scientists, approval letters, and even patient medication guides.
It’s not just for experts. Pharmacists use it to answer patient questions without calling the FDA. Researchers rely on it for systematic reviews. Patients check it to confirm if their medication is truly FDA-approved and when it entered the market. And if you’re comparing generics to brand-name drugs, this is where you find the real approval history.
It’s free, no login needed, and works on any browser. But here’s the catch: if you search the wrong way, you’ll miss key information. Most people get stuck because they don’t understand how the search system works.
How to Search by Drug Name (Brand or Generic)
The homepage has a big search box. That’s your best starting point. Type in any drug name - brand or generic - and hit Enter.
Try searching for lisinopril. You’ll see results for Zestril, Prinivil, Qbrelis, and combination drugs like Zestoretic - all in one list. That’s because the main search looks across brand names, generic names, and active ingredients together.
Now try searching for lisinopril using the A-Z index under “Drug Name.” You’ll only get results for products where lisinopril is the only active ingredient. You won’t see Zestoretic. You won’t see any brand names. That’s a trap. The A-Z index is limited. It’s meant for browsing, not searching. Stick to the main search box unless you’re doing something very specific.
Pro tip: Use the exact spelling. Don’t type “Lisinopril” with a capital L unless you’re sure. The system is case-insensitive, but typos like “lisinopiril” won’t return results. If you’re unsure of the spelling, start with the brand name - it’s easier to remember.
Searching by Active Ingredient
What if you don’t know the brand name? Maybe you’re looking at a pill and only see “5 mg” and a letter on it. You can search by active ingredient.
Go to the main search box and type the ingredient - like metformin, atorvastatin, or sertraline. The results will show every approved product containing that ingredient, including generics and combination pills.
For example, searching for hydrochlorothiazide will return dozens of results - from single-ingredient tablets to combos with lisinopril, olmesartan, or metoprolol. Each result links to the full label and approval documents. This is critical if you’re checking for drug interactions or trying to identify an unknown pill.
Don’t confuse this with the A-Z index again. The A-Z search for active ingredients doesn’t include combination products. Only the main search does.

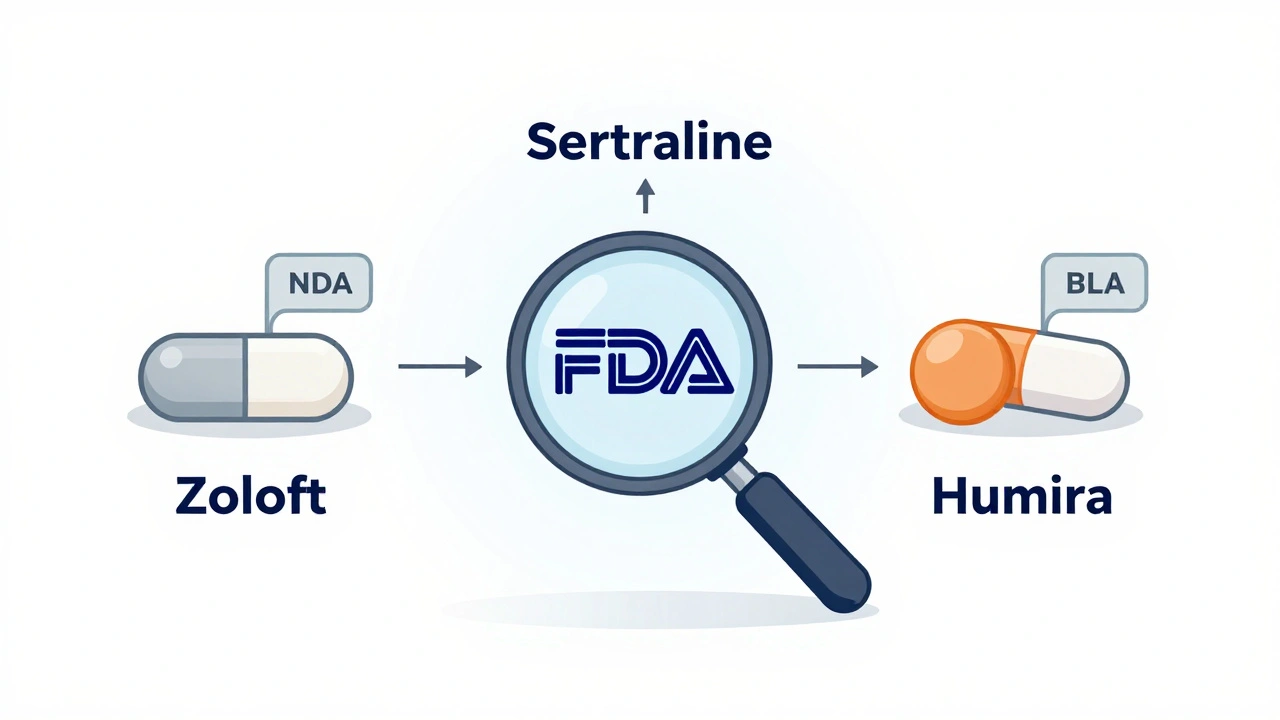
Using Application Numbers (NDA, ANDA, BLA)
If you already have an application number - like NDA 021234 or ANDA 208901 - you can search directly by it. This is the fastest way to pull up a specific drug’s file.
Application numbers are printed on FDA approval letters and sometimes on pharmacy labels. If you’re a healthcare provider or researcher, you might have this number from prior correspondence.
Just paste the full number into the search box. The system recognizes NDA (New Drug Application), ANDA (Abbreviated New Drug Application for generics), and BLA (Biologics License Application). You’ll go straight to the drug’s full regulatory file - no scrolling, no filtering.
Why use this? Because application numbers are unique. There’s only one NDA for Zoloft. Only one ANDA for generic sertraline. No ambiguity. If you need to cite a specific approval in a report or insurance claim, this is your ticket.
Understanding What You’ll See in the Results
After you search, you’ll get a list of matching drugs. Each entry shows:
- Proprietary name (brand name)
- Active ingredient(s)
- Strength and dosage form (tablet, capsule, injection)
- Applicant (manufacturer)
- Approval date
- Application number
- Status (approved, discontinued, etc.)
Click on any drug to open its full record. Here’s where the real value is:
- Labeling - Full prescribing information (the same document doctors use)
- Review Documents - FDA medical and pharmacology reviews explaining why the drug was approved
- Approval Letters - Official letters from the FDA to the company
- Patient Medication Guides - The handouts pharmacies give you
- Correspondence - Letters between the FDA and the manufacturer during review
For drugs approved after 1998, nearly all of this is available. For older drugs, you might only see the label and approval date. But even that’s more than you’ll find on drug company websites or pharmacy apps.
What Drugs@FDA Doesn’t Cover - And What to Use Instead
Drugs@FDA is powerful, but it’s not the only FDA database. Don’t confuse it with these:
- FDALabel - Better for searching specific sections of drug labels (like “Boxed Warning” or “Adverse Reactions”). Drugs@FDA gives you the full label, but FDALabel lets you search within it.
- Orange Book - Shows therapeutic equivalence ratings for generics and patent/exclusivity info. Drugs@FDA doesn’t tell you if a generic is rated AB (therapeutically equivalent).
- Purple Book - Covers only biologics (like Humira, Enbrel). Drugs@FDA includes biologics too, but Purple Book gives more detail on biosimilars.
- Animal Drugs@FDA - Separate database for veterinary medications. Drugs@FDA only covers human drugs.
If you’re checking if a generic is safe to substitute, use the Orange Book. If you need to know if a drug has a black box warning, use FDALabel. But if you want to know when it was approved, who made it, and what the FDA said during review - start with Drugs@FDA.
Real-World Use Cases
Case 1: A pharmacist verifies a patient’s drug history
A patient says they’ve been taking “Zestril” for 12 years. The pharmacist checks Drugs@FDA. The drug was approved in 1987. The label shows it was originally approved as a once-daily tablet. The patient’s current prescription is a generic version approved in 2010. The pharmacist confirms the switch is legitimate and shares the approval date with the patient.
Case 2: A researcher tracks drug approvals over time
A public health student is studying how fast new diabetes drugs reached the market between 2005 and 2020. They use Drugs@FDA to export a list of all metformin, sitagliptin, and semaglutide approvals. They cross-reference with FDA approval letters to note delays in review times.
Case 3: A patient questions a new prescription
A man is prescribed a new generic version of his blood pressure drug. He’s worried it’s not the same. He searches Drugs@FDA by the active ingredient. He sees the generic was approved in 2018, has the same label as the brand, and was reviewed by the same FDA team. He feels confident taking it.
Common Mistakes and How to Avoid Them
- Mistake: Using the A-Z index for brand names. Solution: Always use the main search box.
- Mistake: Searching for “ibuprofen 200mg” instead of just “ibuprofen.” Solution: Leave out strength and dosage - search by ingredient only.
- Mistake: Assuming all generics are listed under their brand name. Solution: Search by active ingredient to see all generics.
- Mistake: Not checking the approval date. Solution: Older drugs may have outdated labels. Always confirm you’re viewing the most recent version.
Pro tip: Bookmark the page. The URL is www.fda.gov/drugsatfda. No login, no ads, no tracking. Just pure FDA data.
Final Thoughts: Why This Tool Is Essential
Drugs@FDA isn’t flashy. It doesn’t have fancy filters or mobile apps. But it’s the closest thing we have to the FDA’s internal records - and it’s public. In a world full of misleading drug ads and sketchy health blogs, this is your anchor to facts.
It’s used by thousands every day. You don’t need a medical degree to use it. You just need to know where to look and what to avoid. Master this one tool, and you’ll never have to guess whether a drug is truly approved or what its real risks are.
Is Drugs@FDA free to use?
Yes. Drugs@FDA is completely free. No registration, no subscription, no hidden fees. It’s a public resource funded by the U.S. government.
Can I find generic drugs on Drugs@FDA?
Yes. All approved generics - including those with ANDA numbers - are listed. Search by active ingredient to see every generic version of a drug, regardless of brand name.
Why can’t I find my drug on Drugs@FDA?
It might not be FDA-approved. Drugs@FDA only includes drugs approved for use in the U.S. It doesn’t include supplements, veterinary drugs, or drugs approved only in other countries. Also, very new drugs may take a few days to appear after approval.
Does Drugs@FDA include drug side effects?
Yes - but only as listed in the official FDA-approved prescribing label. You’ll find side effects under the “Adverse Reactions” section of the drug’s label. For more detailed safety data, you may need to check the FDA’s Adverse Event Reporting System (FAERS), which is separate.
How often is Drugs@FDA updated?
Daily. New drug approvals, label changes, and discontinued products are added every business day. If a drug was approved today, it should appear in the database by tomorrow.



Gene Linetsky
December 3, 2025 AT 19:52Katherine Gianelli
December 5, 2025 AT 01:29Makenzie Keely
December 7, 2025 AT 00:03Joykrishna Banerjee
December 8, 2025 AT 13:05Archie singh
December 9, 2025 AT 03:24parth pandya
December 10, 2025 AT 19:38Francine Phillips
December 11, 2025 AT 12:02Gavin Boyne
December 13, 2025 AT 02:34Charles Moore
December 13, 2025 AT 06:23Vincent Soldja
December 14, 2025 AT 16:41Chloe Madison
December 15, 2025 AT 01:32Kidar Saleh
December 16, 2025 AT 21:55Albert Essel
December 18, 2025 AT 21:52Myson Jones
December 19, 2025 AT 08:57